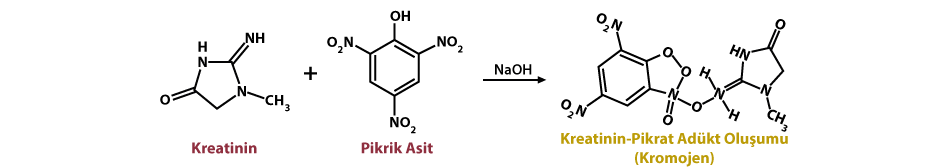
The incidence of non-Hodgkins Lymphoma (NHL) or Malignant Lymphoma (ML) in dogs is reported to be more than 24 per 100,000. Advances in the diagnosis and treatment of ML in dogs not only improve the quality of life of animals but also enable better models in veterinary comparative oncology.
Thymidine kinase (TK) is an intracellular enzyme that plays an important role during pyrimidine synthesis. TK activity increases markedly in the G1-S phase, especially during cell division, and decreases rapidly in the G2 phase. Therefore, high extracellular TK activity reflects high DNA synthesis and cells that die during cell division. Hematopoietic system malignancies are characterized by high cell proliferation. Studies in the veterinary field have shown that serum Thymidine kinase activity is an important marker in the diagnosis, prognosis and monitoring of treatment efficacy in leukaemia, multiple myeloma and malignant lymphoma.
TK activity has been used for years in the diagnosis, prognosis and treatment follow-up of hematopoietic tumours in human oncology, and the first study in the veterinary field was conducted by Nakamura et al. conducted in 1997 in Lymphoma, leukaemia, non-hematopoietic tumours (breast tumour, mastocytoma, anal sac tumour, malignant histiocytosis) and healthy dogs. After the analysis, TC activity in dogs with Lymphoma and Leukemia was significantly increased compared to healthy dogs; in dogs with non-hematopoietic tumours, it was found to be at the same level as healthy dogs. Again in the same study, it was determined that TC Activity is important in the follow-up of the treatment in the analyzes performed before the treatment, at the stage of the disappearance of clinical symptoms, and at the relapse stage.


In another study conducted by Prof. Dr. Hendrik von EULER et al. in dogs diagnosed with ML between 1999-2003, it was reported that TK Activity can be used as a strong marker in the diagnosis of ML disease, especially in determining the prognosis and predicting clinical disease before a recurrence in dogs undergoing chemotherapy. Serum TK Activity was found to be 2 to 180 times higher in dogs with ML disease than in healthy dogs. It was determined that TC activity decreased to normal values in dogs that responded to treatment and whose cancer symptoms disappeared (complete remission), and TC activity increased again before recurrence. In the same study, it was determined that TC activity was correlated with the clinical stages of the disease.
Similar studies have been carried out in cats in recent years, along with studies in dogs. The first study on cats was conducted on a total of 171 cats in the UK and Sweden, published in 2012 and also included in our partner laboratory, Dechra Specialist Laboratories. Of the cats included in the study, 49 were healthy, 33 had lymphoma, 55 had the inflammatory disease, and 34 had non-hematopoietic neoplasia. At the end of the study, it was determined that the serum TC activity was significantly higher in cats with lymphoma compared to the others, and it was reported that high TC activity would strengthen the diagnosis of lymphoma.
Thymidine kinase activity with recent studies;
- In the diagnosis of lymphoma and leukaemia together with other clinical and laboratory findings,
- In evaluating the prognosis,
- In the evaluation of chemotherapeutic success with analyzes performed before, during and after treatment,
- Monitoring chemotherapy and identifying relapse cases before they form,
- It has been used successfully in distinguishing clinical worsening in patients receiving chemotherapy treatment.
References
- Boyé, P. et al. (2019) ‘Evaluation of serum thymidine kinase 1 activity as a biomarker for treatment effectiveness and prediction of relapse in dogs with non-Hodgkin lymphoma.’, Journal of veterinary internal medicine, 33(4), pp. 1728–1739. doi: https://doi.org/10.1111/jvim.15513.
- Bryan, J. N. (2016) ‘The Current State of Clinical Application of Serum Biomarkers for Canine Lymphoma.’, Frontiers in veterinary science, 3, p. 87. doi: https://doi.org/10.3389/fvets.2016.00087.
- von Euler, H. et al. (2004) ‘Serum thymidine kinase activity in dogs with malignant lymphoma: a potent marker for prognosis and monitoring the disease.’, Journal of veterinary internal medicine. United States, 18(5), pp. 696–702. doi: https://doi.org/10.1371/journal.pone.0137871.
- Kayar, A. et al. (2018) ‘Clinical features, haematologic parameters, blood serum biochemistry results and thymidine kinase activity of dogs affected by malignant lymphoma in Turkey’, Japanese Journal of Veterinary Research, 66(4), pp. 227–238. doi: https://doi.org/10.14943/jjvr.66.4.227.
- Larsdotter, S., Nostell, K. and von Euler, H. (2015) ‘Serum thymidine kinase activity in clinically healthy and diseased horses: a potential marker for lymphoma.’, Veterinary journal (London, England : 1997). England, 205(2), pp. 313–316. doi: https://doi.org/10.1016/j.tvjl.2015.01.019.
- Nakamura, N. et al. (1997) ‘Plasma thymidine kinase activity in dogs with lymphoma and leukemia.’, The Journal of veterinary medical science. Japan, 59(10), pp. 957–960. doi: https://doi.org/10.1292/jvms.59.957.
- Selting, K. A. et al. (2016) ‘Thymidine Kinase Type 1 and C-Reactive Protein Concentrations in Dogs with Spontaneously Occurring Cancer.’, Journal of veterinary internal medicine, 30(4), pp. 1159–1166. doi: https://doi.org/10.1111/jvim.13954.
- Taylor, S. S. et al. (2013) ‘Serum thymidine kinase activity in clinically healthy and diseased cats: a potential biomarker for lymphoma.’, Journal of feline medicine and surgery. England, 15(2), pp. 142–147. doi: https://doi.org/10.1177/1098612X12463928.