Author: Sude Sak
Adipose tissue is a type of connective tissue that plays an important role in energy metabolism. It includes adipocytes. Adipocytes, which are closely associated with small blood vessels, are found singly or in groups, often within lobules surrounded by fibrous septa. Adipose tissue contains different cell types. Only one-third of the tissue consists of adipocytes. The rest form fibroblasts, macrophages, stromal cells, monocytes, and preadipocytes.
Adipose tissue is a critical regulator of systemic energy homeostasis by acting as a calorie reservoir. Under nutrient-excess conditions, adipose tissue stores excess nutrients in the form of neutral lipids, while under nutrient-deficient conditions, it provides nutrients to other tissues through lipolysis.
It is a dynamic tissue that is involved in the synthesis and storage of lipids in order to meet the energy needs of the body, and that constantly changes in volume in terms of cell number and size. Excess energy is stored in lipid droplets in the form of triglycerides.
Triglycerides, which are the most concentrated form of metabolic energy storage in humans, store twice as much energy as carbohydrates and proteins.
Simultaneously, various stromal vascular cells in adipose tissue undergo numerical and/or functional changes, contributing to the maintenance of adipose tissue’s function as an energy store and endocrine organ.
There are three types of adipose tissue
White adipose tissue (WAT) is the predominant type of fat in the human body. WAT has several biological functions, including energy storage, prevention of heat loss, protection of vital organs, and hormone secretion. Some hormones include leptin, adiponectin, and resistin.
Beige adipocyte tissue, the third and most recent type of adipocyte, can emerge in VAT in response to thermogenic stimulation, a process known as the browning of WAT. Recent research suggests that the browning of WAT deserves more attention and that therapies that target the browning of WAT can help reduce obesity. Beige adipocytes reside within WAT and expend energy to generate heat during cold exposure (called cold-induced thermogenesis). It is well known that activated beige adipose tissue can stimulate weight loss and promote resistance to obesity, making it an attractive therapeutic target tissue. Ageing is the primary risk factor for obesity and is associated with loss of beige adipose tissue, suggesting that loss of energy expenditure capacities may contribute to an obesity-prone phenotype with increasing age.
Almost every mammal has brown adipose tissue (BAT). In newborns and hibernating mammals, brown adipose tissue is especially abundant. It is also present in adults and is metabolically active, but its prevalence decreases with age. The primary function of this gland is thermoregulation. The mitochondria of BATS cells are observed to be brown in colour due to the presence of large amounts of cytochromes. It is also called the hibernating gland because these fat stores function for the animal during its awakening from hibernation.
Energy Metabolism
The metabolism and mobilization of lipids are under the control of adipose tissue. Lipogenesis is the process through which carbohydrates are converted into fatty acids, promoting the biosynthesis of triglycerides (TG) and the expansion of lipid droplets within adipocytes. Conversely, lipolysis breaks down TG into free fatty acids (FFA) and glycerol, which can be oxidized or released.
The uptake of circulating FFAs by the liver, muscles, and other tissues constitutes a primary pathway for lipid mobilization. Both the pathways of lipogenesis and lipolysis are highly sensitive to nutritional factors and hormones such as insulin, norepinephrine, and glucagon. As a result, the intricate regulation of these processes is essential for maintaining systemic energy homeostasis and insulin sensitivity.
Lipolysis and Lipogenesis
Lipogenesis is the term used to describe the synthesis of triglycerides and fatty acids from acetyl coenzyme A. In contrast, lipolysis involves the breakdown of triglycerides, leading to the formation of fatty acids. The key distinction between these two processes lies in their fundamental nature. Specifically, lipolysis is centered around the hydrolysis of fats and various lipid molecules, resulting in the production of fatty acids. Conversely, lipogenesis entails the creation of fatty acids and triglycerides from substrates like acetyl coenzyme A and other precursors.
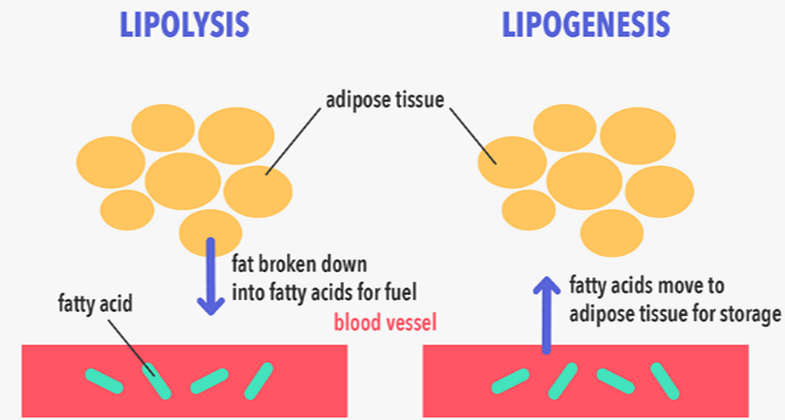
Adipose tissue serves as a crucial energy storage reservoir, housing triglycerides (TGs) that are released as fatty acids through processes called lipogenesis and lipolysis, respectively.
The systemic intake of food triggers the activation of the lipogenic pathway, encouraging TG storage in adipose tissue. Conversely, fasting initiates the lipolytic pathway, prompting the breakdown of TGs and the subsequent release of fatty acids from adipose stores. This intricate balance involves lipogenesis, a process of creating fresh fatty acids from acetyl-coenzyme A (acetyl-CoA), and TG synthesis.
The metabolism of glucose generates acetyl-CoA, a pivotal component for fatty acid synthesis. This process also boosts the expression of acetyl-CoA carboxylase, the rate-controlling enzyme in lipogenesis, and triggers the release of pancreatic insulin, further propelling lipogenesis. In essence, adipose tissue functions as an energy reservoir, effectively mitigating fatty acid fluxes and averting lipotoxicity and insulin resistance. This tissue also manages the clearance of plasma TGs, averting their accumulation in other bodily tissues.
Consequently, the adipose tissue’s lipid storage capacity plays a pivotal role in systemic insulin resistance and the infiltration of lipids into organs such as the liver and muscles. On the contrary, lipolysis entails the catabolic breakdown of stored TGs within adipocytes, liberating free fatty acids and glycerol.
Starvation triggers lipolysis, yielding glycerol for hepatic gluconeogenesis and free fatty acids for oxidation, catering to the energy requirements of other organs. When fatty acids abound and carbohydrates are scarce, the liver can further metabolize fatty acids to create ketone bodies, a process termed ketogenesis, which serves as an energy source for the brain. This dynamic interplay between lipogenesis and lipolysis is pivotal for maintaining systemic energy equilibrium and insulin sensitivity. Overall, adipose tissue’s multifaceted functions underscore its significance as an energy reservoir and regulator within the body’s energy homeostasis.”
Adipose tissue acts like an endocrine organ
White adipose tissue emerges as a pivotal endocrine organ, playing a dual role in lipid storage or release and energy equilibrium by engaging in the secretion of essential adipokines. Among these, adipocytes secrete polypeptides like leptin, resistin, and adiponectin, which orchestrate a delicate balance crucial for glucose and lipid metabolism homeostasis. The intricate interplay of these adipocytokines emanating from adipocytes fundamentally contributes to sustaining optimal energy levels.
Leptin, a key player, responds to factors such as excessive energy intake, insulin levels, and glucose levels, resulting in varying production rates. Conversely, fasting, exposure to cold, β-adrenergic agonists, and testosterone lead to decreased leptin secretion. Adiponectin, a collagen-like plasma protein synthesized within adipose tissue, plays a significant role. While its concentration is higher in subcutaneous white adipose tissue, visceral white adipose tissue and hypertrophic adipocytes are inversely correlated with circulating adiponectin levels. Weight loss and periods of hunger trigger an increase in plasma adiponectin levels, which in turn activate glucose utilization within muscles. This cascade drives enhanced fatty acid oxidation in the liver and muscles, subsequently curbing glucose production due to inhibited gluconeogenesis.
The regulation of adaptive thermogenesis
Thermogenin (uncoupling protein 1, or UCP1), a distinctive molecule inherent to cold-induced thermogenesis, assumes a crucial role as it is selectively expressed within brown adipose tissue. It orchestrates a remarkable metabolic shift by diverting oxidative phosphorylation away from ATP synthesis, and channeling the energy towards heat generation instead of ATP production.
Instances of cold exposure and heightened nutritional intake trigger a surge in brown adipose tissue activity, accompanied by elevated expression levels of norepinephrine and UCP1, which emanate from the central nervous system. Notably, a repertoire of agents, including β-adrenergic antagonists, thyroid hormones, insulin, and cAMP analogues, also contribute to the augmentation of UCP1 expression.
In response to cold and nutrient availability, sympathetic nerve activity intensifies within adipose tissue. Noradrenaline binds adeptly to β-adrenergic receptors, thereby instigating a cascade of molecular signals that culminate in the hydrolysis of triglycerides. The ensuing liberation of fatty acids plays a dual role, not only energizing UCP1 but also fueling thermogenesis in cold-induced thermogenic pathways, with glucose serving as the exclusive carbon source for degradation.
Notably, the activation extends to beige cells, further enhancing the thermogenic response. Consequently, this orchestrated mechanism precipitates a surge in whole-body energy expenditure while concurrently reducing body fat mass. In essence, the interplay of these intricate processes orchestrates a metabolic symphony that elevates energy expenditure and diminishes body fat mass.
This article has been prepared from the presentation of our student Sude Sak.
References
Bódis, K, Roden, M (2018). Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest., 48:e13017. DOI: 10.1111/eci.13017
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB (2016). Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne), 7:30. DOI: 10.3389/fendo.2016.00030.
Mermer M, Tek NA (2017). Adipoz doku ve enerji metabolizması üzerine etkileri. Sdü Sağlık Bilimleri Enstitüsü Dergisi, 8(3): 40-46. DOI: 10.22312/sdusbed.292229
Wang Z, Wang QA, Liu Y, Jiang L (2021). Energy metabolism in brown adipose tissue. FEBS Journal, 288(12): 3647-3662. DOI: 10.1111/febs.16015.
Zhu Q, Glazier BJ, Hinkel BC, Cao J, Liu L, Liang C, Shi H (2019). Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues. Int J Mol Sci., 20(11): 2707. DOI: 10.3390/ijms20112707.
Published on: 10 August 2023Edited on: 10 August 2023