The existence of molecular chirality in the substances in the universe and the stereoisomer properties of molecules with stereogenic centres have been very influential in the development of organic chemistry and related sciences. The reason why chirality is important as biological activity is that molecular symmetry dominates biological events.
Although chirality is not essential for bioactivity, there are great differences in the activities of enantiomers in bioactive molecules with stereogenic centres such as drugs, flavourings, and food additives. The molecular components of living organisms are mostly chiral and these molecules have a dominant role in their interaction with bioactive substances. Receptors or free nerve endings enable all living systems to recognize and perceive all changes in the external environment they live in, such as chemical, physical, electrical, etc. that occur in their internal environment. Regardless of their functions in organisms and structures, the common point of all receptors is that they all consist of chiral molecules. Amino acids, DNA, carbohydrates and enzymes are the basic chiral structures found in living things. It is desired that the receptors behave differently in binding to the stimulant molecules, that is, they are enantioselective. For this reason, the synthesis of active substances with chirality characteristics is important because they are pharmaceutical raw materials. In drugs, one of the enantiomers exhibits desirable behaviours and beneficial pharmacological properties, while the other enantiomer may exhibit harmful pharmacological properties in general.
Today, pure compounds with enantiomeric behaviour are needed more than ever for the production of drugs and nutritional additives used in human, animal and plant health, and for the development of material science and industry such as liquid crystal and polymer. When the structures of living things and their activities were investigated, it was determined that since single isomers are target-selective in terms of biological effect, both enantiomers of the optically active substance are stronger and more reliable than having the same amounts in a mixture (racemic). As a result, it has been focused on the development of drugs consisting of a single enantiomer with a single isomer of the active substance. While one of the enantiomers in the racemic mixture of drugs with chiral compounds is physiologically beneficial, the other enantiomer can cause serious harm. Therefore, the definition of chiral structures in the pharmaceutical industry is very important. For all these reasons, the need for the development of new asymmetric synthesis methods has increased. Thus, studies on this subject have gained momentum in recent years.
Stereochemistry
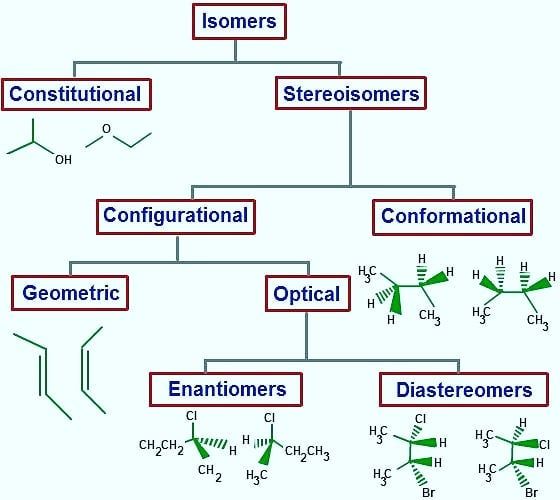
The branch of science that examines the bonding structures of atoms in the molecule and their arrangement in space in three dimensions is called stereochemistry. He studies how atoms are arranged in their relative positions in space. Isomerism is a discipline that studies the arrangement of molecules in space. Isomers are two or more different chemical compounds that have different bonding structures between their atoms but can be represented by the same molecular formula. These are compounds that have the same molecular formula but different arrangements of atoms. At the same time, the chemical properties of isomer compounds are also different.
Stereoisomers
The covalent bonds and functional groups of a biomolecule are very important in the function of that molecule. The three-dimensional form of the molecule is formed by atoms. Carbon compounds exist in the form of stereoisomers. Their atomic numbers are the same, but their arrangement in space is different, and they also show different physical properties. Molecular interrelationships between biomolecules are invariably stereospecific, which creates specific stereochemistry in the molecules. The arrangement of atoms is called configuration, these can be cis, trans configurations, or configurations of optical stereoisomers. These are referred to as three-dimensional structures in stereochemistry. A carbon atom is asymmetric with 4 different atoms or groups. A molecule containing one or more asymmetric carbons is also asymmetric.
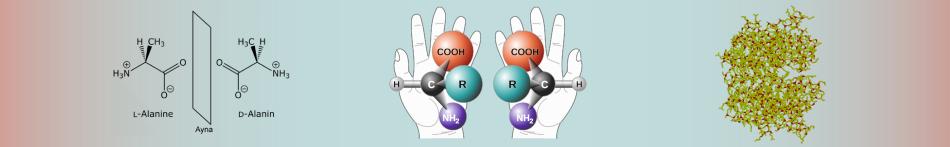
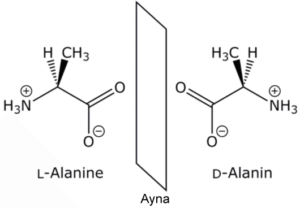
Enantiomers cannot be converted to each other. This is because enormous energies are required to break the covalent bonds and displace the atoms. Enantiomers have the same chemical properties as long as they are not in non-chiral environments. For example, there are two enantiomers of alanine, an amino acid, and their physical properties such as melting point, boiling point and solubility are the same, but if the enantiomers are mixed, the physical properties of the product such as melting, freezing point and solubility will be different, but their chemical properties will not change. If the ratio of enantiomers in a mixture is desired, the chromatographic and spectroscopic properties change by showing an externally asymmetric effect. Thus, the enantiomers act differently from each other and their analysis is done.
Enantiomers
It is an asymmetric mirror image of the optically active substance (chiral substance) and has two enantiomers. It is formed by the bonding of the chiral molecule to four different groups or the carbon group of the atom with σ-bonds. Although the carbon atom is the asymmetric centre of the molecule, such molecules have a pair of stereoisomers with different spatial structures. They do not conflict with mirror images. Their optical rotation angles are the same in numbers but opposite in sign. The melting and boiling points, densities, and all physical properties of the two symmetric enantiomers are similar. Their chemical properties are the same in non-chiral environments, they react at a similar rate and form to form similar substances. Optical rotation angles are the same numerically but opposite in sign. It is possible to separate them from each other with advanced chemical analysis methods. Since most living molecules are enantiomers, there is an obvious difference due to the effects of the two symmetric enantiomers on living things, including humans.
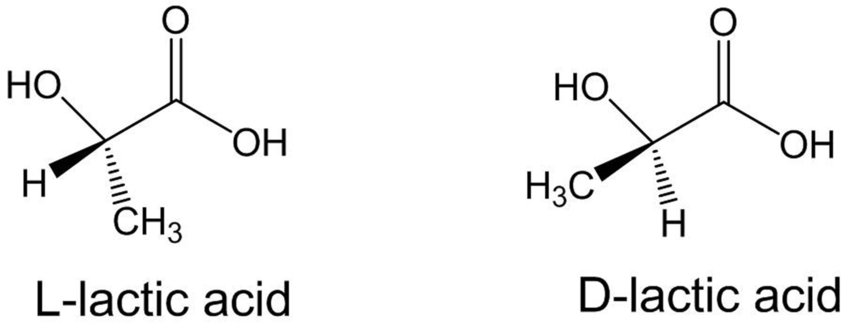
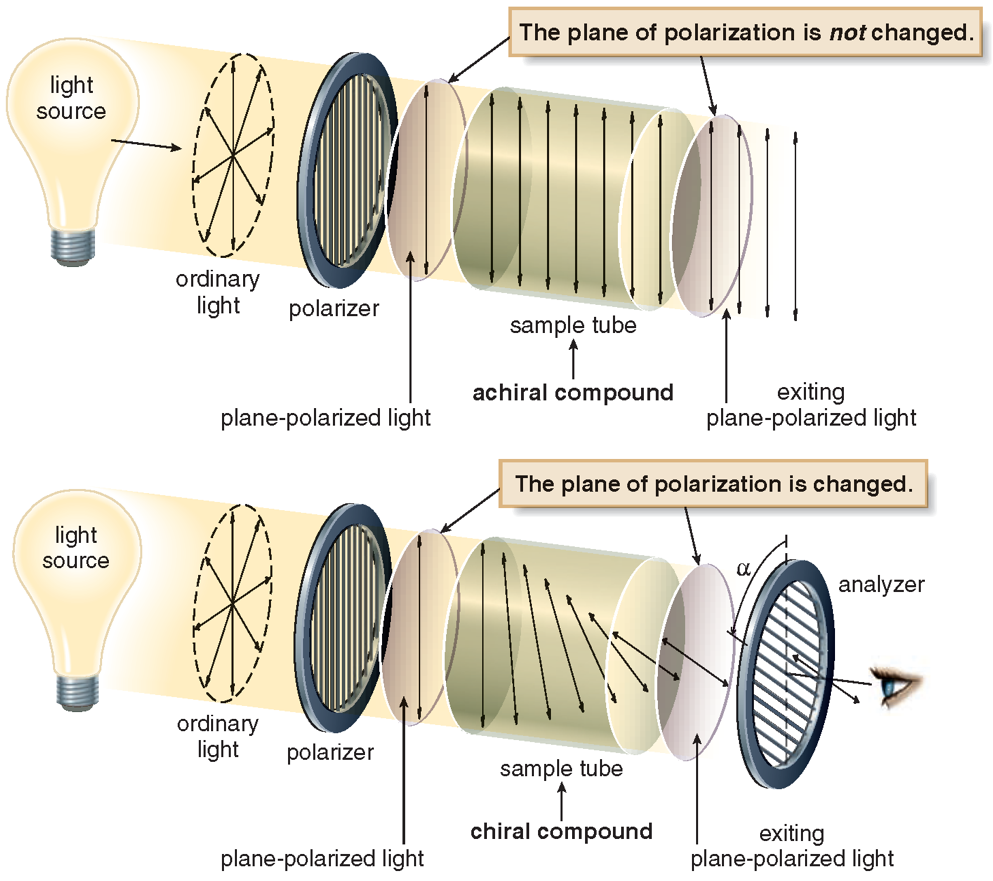
One of the physical properties of enantiomers is that the polarized beam has the ability to rotate the vibrational plane. For this reason, enantiomers are also called optical isomers. One of the optical isomers rotates the propagation of the polarized beam to the right, and the other to the left. Normal light emanating from a single source is a vibratory phenomenon that oscillates (spreads) in all directions and in all planes. Wave motion follows a path perpendicular to the direction of the light. Polarized light is light that vibrates in a single perpendicular plane and is free of wave vibrations. Enantiomer (symmetric) molecules make a definite spin when they encounter light, and when their mirror images are in the opposite direction, the spin is balanced and the spin of the light is reset. The net rotation of a single non-mirrored enantiomer in solution is not reset. Therefore, the rotation of polarized light is reset to zero in racemic mixtures. The specific rotation angles of optically active substances can be measured with a polarimeter. The enantiomer that rotates the vibrational plane of polarized light to the right is called dextrorotatory, or the enantiomer that rotates to the right, and the enantiomer that rotates to the left is called levorotatory or left-handed.
Chirality
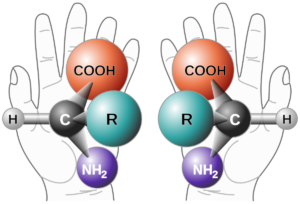
Chirality is a geometric property. If we explain this with an example from daily life if an item does not coincide with its mirror image, it is chiral, and if it coincides with its mirror image, it is not chiral. The characteristic of the chirality molecule is that the mirror image of the arrangement of the atoms of the molecule in space does not overlap. The chiral molecule has an asymmetric centre. Besides single chiral, there are chiral with more than one asymmetric centre. These are called multichiral structures. A molecule, however, may not exhibit chiral properties. Even if four different groups are attached to the sp3 hybridized carbon atom in a compound, it is chiral. If the asymmetric carbon atom is the centre of 4 different atoms or groups of atoms, it is called a stereogenic centre. Chiral compounds are asymmetric molecules due to the absence of an intramolecular symmetry plane and have two configuration isomers whose mirror images do not overlap each other. These two isomers are called enantiomers and these structures are enantiomeric to each other.
Nomenclature and properties of chirality
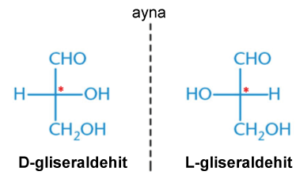
The R and S rules, or Chan-Ingold-Prelog (CIP), is used to name two enantiomers with opposite configurations. This naming is done within the framework of some rules. Simply put the smallest group is placed behind the stereogenic carbon atom and the remaining three groups are decided based on their order of precedence by looking at their right (R) or left (S) orientation. However, in the event that a decision cannot be made with this rule, and if the first bonded atom is the same, the atoms that continue in the second or third order are examined and a decision is made.
After passing through the chiral molecules, plane-polarized light ceases to be a plane, rotates and forms an angle. This is called optical activity. On one side the angle becomes +α° (clockwise) and on the other side, the angle becomes -α° (counterclockwise). For example, at the same concentration and experimental conditions (S)-carvone rotates plane-polarized light +10° clockwise, while (R)-carvone rotates plane-polarized light -10° counterclockwise.
The biological significance of chirality
The existence of chiral molecules and the existence of molecules with stereogenic centres have also made great contributions to organic chemistry and other branches of organic chemistry.
Chirality is not required for biological activities to occur, but the enantiomeric activity of biologically active molecules with stereogenic centres is different. For example, it is found in biologically active substances such as food additives, fragrances and flavours, pesticides, herbicides and medicines. Living organisms contain largely chiral molecules in their components, and these chiral molecules play a dominant role in the mutual action of bioactive substances. All physical, chemical, and electrical differences experienced in living systems are perceived by free nerve endings or receptors with sensory receptors. Whatever their physiological function, the common feature of receptors is that they are chiral. Thanks to this feature, excitatory molecules behave enantioselectively during binding.
Many pharmaceuticals and chemical compounds used in disease treatment are composed of chiral enantiomers with the same physical and chemical properties in racemic structure. In the pharmaceutical industry, one of the enantiomers is desired curative, therapeutic, etc. While the other enantiomer may be inactive, it may have a beneficial effect or may cause harmful side effects. Since there is a need for chiral compounds of enantiomeric purity in the pharmaceutical industry, it is important to develop appropriate separation methods and establish new strategies in the purification processes of chiral molecules from racemic mixtures. Many optical resolution methods are used to separate racemic compounds. However, classical methods require a long process. This situation creates high costs, and a low amount of optically active compound is obtained in return, therefore it is not preferred in the pharmaceutical industry. Membrane-based chiral separation processes are economical compared to other separation processes due to their high efficiency and energy saving. In addition, this method is preferred because the scale-up step is easy.
Chiral molecules
Enzymes: Enzymes, which are chiral compounds, play a role in the realization of many reactions with their cofactors and coenzymes. They act as biocatalysts. Enzymes, with their enantioselectivity, are catalysts for asymmetric synthesis in oxidation-reduction reactions.
Amino acids: The functions of proteins depend on their interactions with other molecules through chiral structures. Homochirality of amino acids is necessary for the gene to specifically code for protein because L- and D-amino acids form three-dimensional protein structures. For example, the amino acids alanine and serine each have a stereogenic centre marked with an asterisk (*). In nature, they occur as natural enantiomers. Proteins, on the other hand, are actually a single enantiomer, despite having many chiral centres.
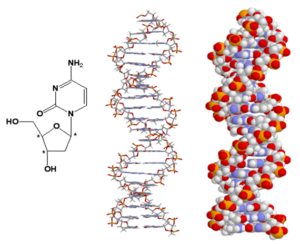
DNA: One of the basic building blocks of living things, the double-stranded DNA, in which genetic information is found, is also a chiral molecule. For example, the nucleic acid cytosine in DNA has three stereogenic centres indicated by an asterisk, and one can speak of chirality. Like proteins, DNA is a single enantiomer despite having many stereogenic centres.
Carbohydrates: They are examined in three subgroups monosaccharides, disaccharides, and oligosaccharides. Carbohydrates are also in the class of chiral molecules.
Lipids: Lipids are chiral molecules, which are the most important units of the cell membrane. He earned this title thanks to his optical activity. The enantiospecific interactions involved here change the properties of the cell, which can significantly affect the role of membrane-bound proteins.
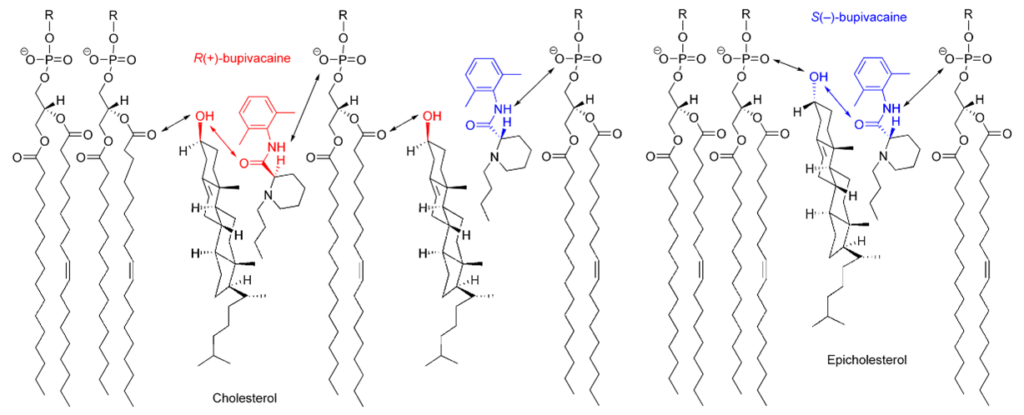
Pharmaceuticals: The importance of chirality in the pharmaceutical industry is an undeniable fact. The enantiomer, which is a mirror image of each other, behaves as two different compounds in the chiral environment, in addition to the difference between the directions of rotation of the vibrational plane of the polarized light. Therefore, their chemical properties in the chiral environment are also different. Since enantiomers have chiral properties in living organisms and structures, they can act in two different ways on these living organisms and structures. In other words, enantiomers can have opposite effects on each other. For example, (S)-(-)-propranolol has been described as a β-blocker in the treatment of heart disease, but its enantiomer (R)-(+)-propranolol acts as a contraceptive. Therefore, the enantiomeric purity of this compound is very important in clinical use. The concept of chirality is very common in pharmaceuticals. Pravastatin sodium for cardiovascular diseases, sertraline hydrochloride for central nervous system diseases, salmeterol for respiratory system diseases, clopidogrel bisulfate for haematology diseases, esomeprazole magnesium for digestive system diseases, potassium clavulanate as an antibiotic can be given as examples.
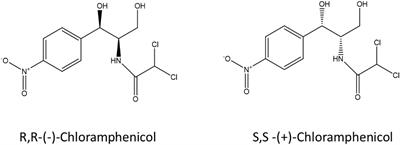
While 56% of the drugs used by the patients are chiral molecules, 88% are found as racemic mixtures. However, when using racemic drugs, it is necessary to use two layers of the racemic mixture in order to fully absorb the unit amount of active substance. For example, (R,R)-chloramphenicol shows antibacterial properties, while (S,S)- chloramphenicol shows inactive properties. Therefore, using racemic mixtures is uneconomical as it wastes half of the resources. Therefore, a single enantiomer always shows more biological activity than a racemic mixture.
As a result, chiral molecules are of great importance for living things. After the discovery of the concept of chirality, studies on naming them were made easier in the expression of these molecules and the understanding of the structure of chiral molecules was facilitated. The right or left orientation of chiral molecules is the factor that changes the way it acts. This is due to the fact that the two enantiomers act differently from each other. Contributing to the formation of the basic building blocks of living things is one of the most important effects of chirality. The contribution of chirality to the pharmaceutical industry is great. Almost half of the drugs on the market are chiral and approximately 50% of them are enantiomer mixtures. From the past to the present, many chiral drugs have been produced, and while most of them have been beneficial, some have been harmed. Chirality is a concept that provides convenience in the field of drug production, if a solution is found for these damages and if the harmful enantiomers can be prevented from showing side effects in the living thing. Biological compatibility, metabolism rate, metabolites, secretion, potency and receptor selectivity, transporters and enzymes, and toxicity of the two enantiomers of chiral drugs can be different. The use of single enantiomer drugs can potentially lead to easier pharmacokinetics and more selective pharmacological profiles, despite reduced drug interactions and different metabolism rates of different enantiomers. Single isomer chemicals can be preferred to racemic chemicals because they are more effective, use less quantity and are more economical in terms of the production process.
This article is a part of the homework written by İremgül Çelikyurt and Melis Tarkan, our Organic Chemistry students for the Fall Semester of the 2020-2021 Academic Year.
References
- Alija K. 2016. Biyolojik Aktif Bileşiklerde Kiralitenin Önemi. Lisans Araştırma Projesi. T.C. Ankara Üniversitesi Eczacılık Fakültesi, Farmasötik Kimya Anabilim Dalı.
- Anonim. 2020. Chirality and Optical Activity. Erişim adresi: [http://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/chirality.html], Erişim tarihi: 17.12.2020
- Anonim. 2020. Chirality-Biological Molecules (DNA). Eriişm adresi : [http://iverson.cm.utexas.edu/courses/310N/MOTD%20Fl05/Chiral.html#:~:text=Because%20the%20building%20blocks%20have,%22right%2Dhanded%22%20helix.&text=Like%20DNA%2C%20proteins%20are%20chiral,acid%20building%20blocks%20are%20chiral]; Erişim tarihi: 17.12.2020
- Anonim. 2020. Chiral Drugs. Reading to learm activity (4). Erişim adresi: [https://cd1.edb.hkedcity.net/cd/science/chemistry/s67chem/pdf/sRL_4_Chiral_drugs.pdf]; Erişim tarihi: 17.12.2020
- Anonim. 2020. Stereochemistry. Erişim adresi: [http://employees.csbsju.edu/cschaller/principles%20chem/stereochem/stereo_carbs.htm]; Erişim tarihi:18.12.2020
- Cheung D. 2007. Teaching Chemistry Through The Jigsaw Strategy. Quality Education Fund, Hong Kong. 2-5.
- Çakmak R. 2008. PirKLe –Tip Kiral Kolon Kromotografisi Yöntemiyle Biyolojik Öneme Sahip Kiral Aminlerden (±)-Β-Metilfeniletilamin’in Rezolüsyonu. Yüksek Lisans Tezi. T.C. Harran Üniversitesi Fen Bilimleri Enstitüsü.
- Çetin A. 2010. Kiralite ve Biyolojik Aktivite. 24. Ulusal Kimya Kongresi, Zonguldak Karaelmas Üniversitesi. Erişim adresi: http://kimyakongreleri.org/2010/2010-701.pdf; Erişim tarihi: 12.11.2020
- Demelezi V. 2018. Tetraoksokaliks[2]Aren[2] Triazin-Bazlı Kiral Bileşiklerinin Sentezi Ve Enantiyoselektif Reaksiyonlarda Katalizör Olarak Kullanımı. Yüksek Lisans Tezi. T.C. Necmettin Erbakan Üniversitesi Fen Bilimleri Enstitüsü.
- Gal, J. 2006. Chiral Drugs from a Historical Point of View. In Chirality in Drug Research (eds R. Mannhold, H. Kubinyi, G. Folkers, E. Francotte and W. Lindner). https://doi.org/10.1002/9783527609437.ch1
- İnaki M, Liu J, Matsuno K. 2016. Cell chirality: its origin and roles in left–right asymmetric development.Phil.Trans. R. Soc. B371: 20150403. http://dx.doi.org/10.1098/rstb.2015.0403
- Karaküçük A. 2006. Kimyasal ve Biyoteknolojik Yöntemlerle Kiral Yapıların Sentezleri. Doktora Tezi. Selçuk Üniversitesi Fen Bilimleri Enstitüsü.
- Lalitha S., Sampath Kumar A., Stine K. J., Covey, D. F. 2001. Chirality in Membranes: First Evidence that EnantioselectiveInteractions Between Cholesterol and Cell Membrane Lipids Can Be a Determinant of Membrane Physical Properties. Journal of Supramolecular Chemistry, 1(2): 53-61
- Serpek, B. 2015. Organik Kimya. Nobel Akademik Yayıncılık
- Smith JG. 2010. Organic Chemistry, 3rd Edition, McGraw-Hill.
- Smith JG. 2012. General, Organic, & Biological Chemistry 2nd Edition, McGraw-Hill.