Author: Doğa İsmailoğlu
Myocardial metabolism refers to the complex biochemical processes that occur within the heart muscle, or myocardium, to provide the energy needed for its continuous and vigorous contraction.
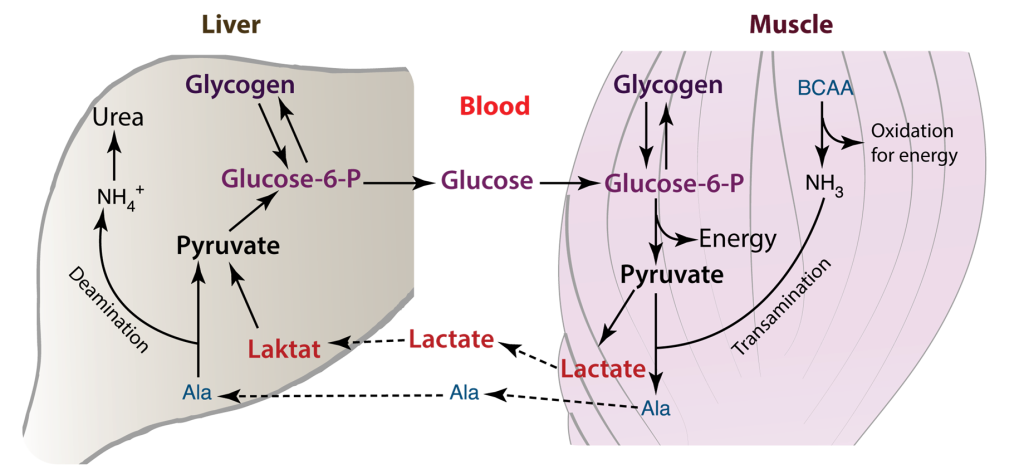
The heart has the highest metabolic demand of all our organs. Thus, myocardial metabolism is an area of interest for biochemists. The heart requires a sufficient supply of ATP to facilitate muscle contraction, sarcomere relaxation, and the active transport of ions across the cell membrane, as seen in processes like Na+/K+-ATPase. This demand for energy is contingent on the availability of oxygen. When an adequate supply of oxygen is present, glycolysis occurs aerobically, proceeding to the tricarboxylic acid (TCA) cycle and electron transport. In cases where oxygen is limited or absent, anaerobic glycolysis ensues, stopping at the pyruvate stage, which is then converted to lactate. This lactate is subsequently transported to the liver, where it is transformed into glucose through gluconeogenesis. In the cardiac muscle, the Cori cycle can become active in situations of heightened energy demand or stress, such as during exercise or in specific disease conditions.
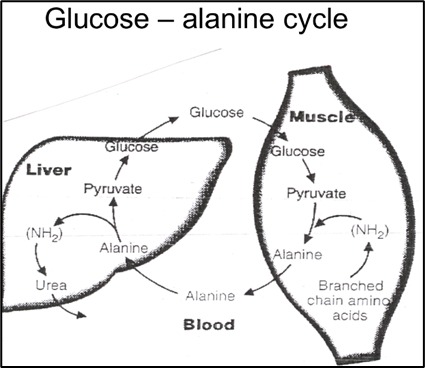
Cori Cycle plays a vital role in mycocardial metabolism
The Cori cycle plays a vital role in upholding energy production and glucose balance within cardiac muscle and other tissues, particularly when energy demand surges or oxygen availability is limited. In cardiac muscle, the Cori cycle comprises the subsequent stages.
Glycolysis: Glucose undergoes glycolysis, which consists of a sequence of chemical reactions within the cytoplasm of cardiac muscle cells. This process yields pyruvate and a modest quantity of ATP.
Lactate generation: In specific scenarios, such as when oxygen is scarce or during periods of elevated energy requirements, pyruvate is transformed into lactate via anaerobic glycolysis. Subsequently, lactate is released into the bloodstream.
Lactate Uptake: Lactate generated within the cardiac muscle has the capacity to be absorbed by various tissues, including the liver, skeletal muscles, or even other cardiac muscle cells. In these tissues, it can serve as an energy source or be reconverted into glucose.
Glucose Regeneration: Within the tissues that receive lactate, such as the liver, there exists a process called gluconeogenesis, which can convert lactate back into glucose. This glucose is subsequently released into the bloodstream and taken up by cardiac muscle cells, where it is utilized as an energy source, effectively concluding the Cori cycle.
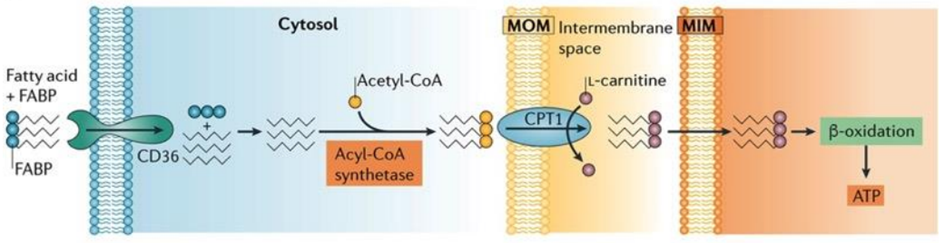
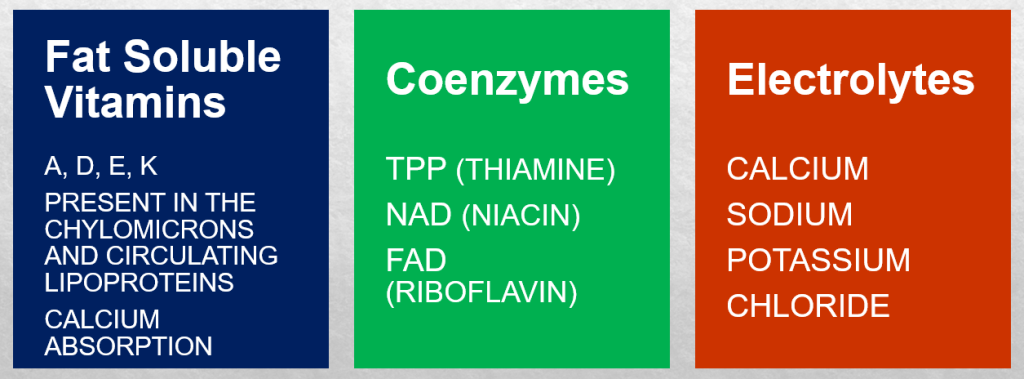
The heart’s energy requirements are also influenced by the availability of substrates beyond just oxygen. About 70% of the cardiac ATP is generated through the beta-oxidation of fatty acids, which serve as the primary energy source for an adult heart. These fatty acids originate from chylomicrons and result from the hydrolysis of triglycerides by lipoprotein lipase. Carbohydrates, on the other hand, serve as the energy source for the fetal heart and for an adult heart under stressful conditions, such as during ischemia.
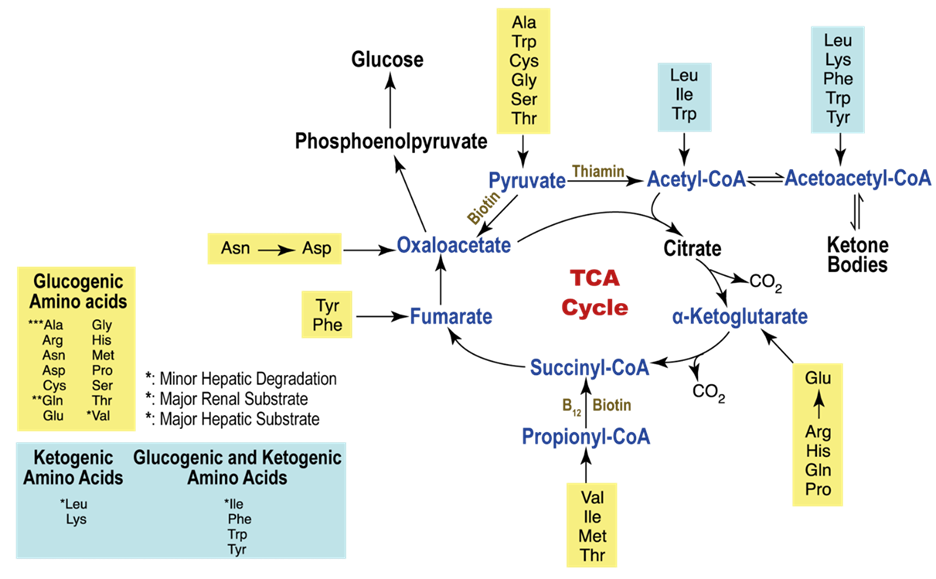
In unusual situations like starvation, amino acids, and ketone bodies can also be utilized to produce ATP in metabolism. Apart from substrates, there is an additional requirement for nutrients to support ATP synthesis, which includes fat-soluble vitamins such as A, D, E, and K. These vitamins are present in chylomicrons and circulating lipoproteins and are released through the action of lipoprotein lipase. Vitamin D plays a crucial role in calcium absorption from the intestines. Coenzymes like TPP (thiamine), NAD (niacin), and FAD (riboflavin), as well as electrolytes such as calcium, sodium, potassium, and chloride, are essential for ATP production as well.

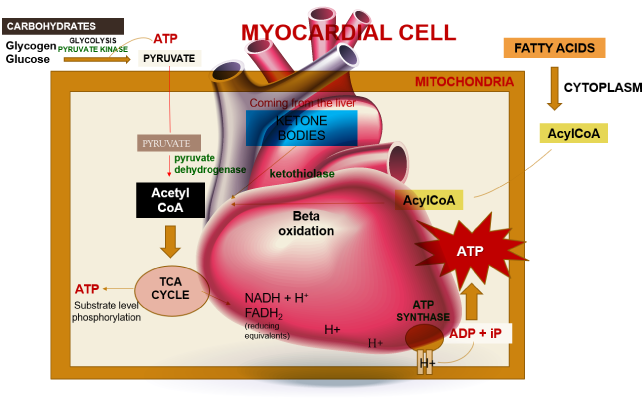
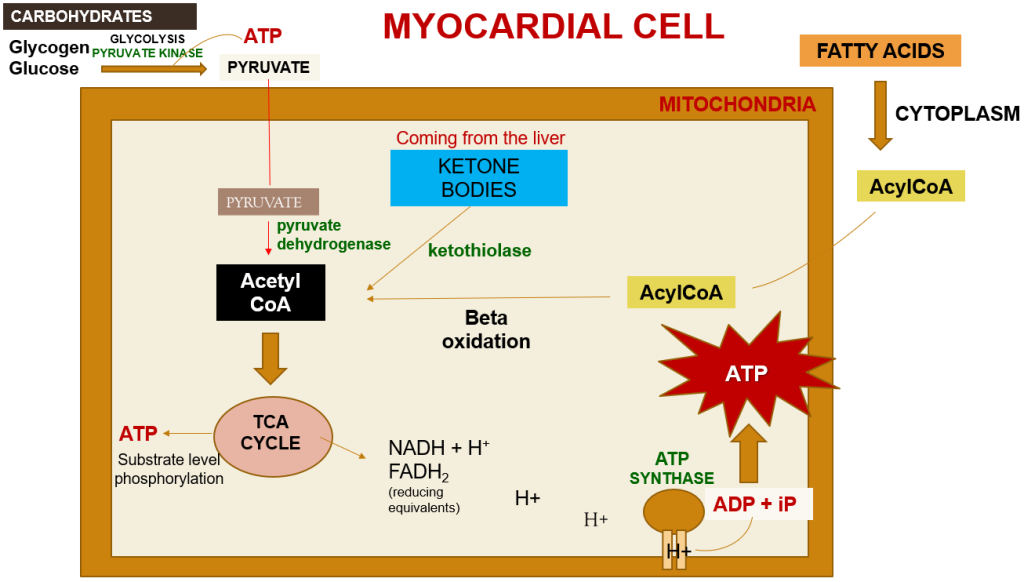
Within a myocardial cell, myocardial metabolism, glucose from the bloodstream, and glycogen stored in the myocardium go through glycolysis with the assistance of the pyruvate kinase enzyme, leading to the production of pyruvate. During this process, substrate-level phosphorylation takes place, yielding a small quantity of ATP. This pyruvate is then transported into the mitochondria, where it is converted into acetyl-CoA by the pyruvate dehydrogenase enzyme complex. Additionally, acetyl-CoA can also be produced from ketone bodies, synthesized by the liver but not utilized, and the ketothiolase enzyme plays a role in this process.
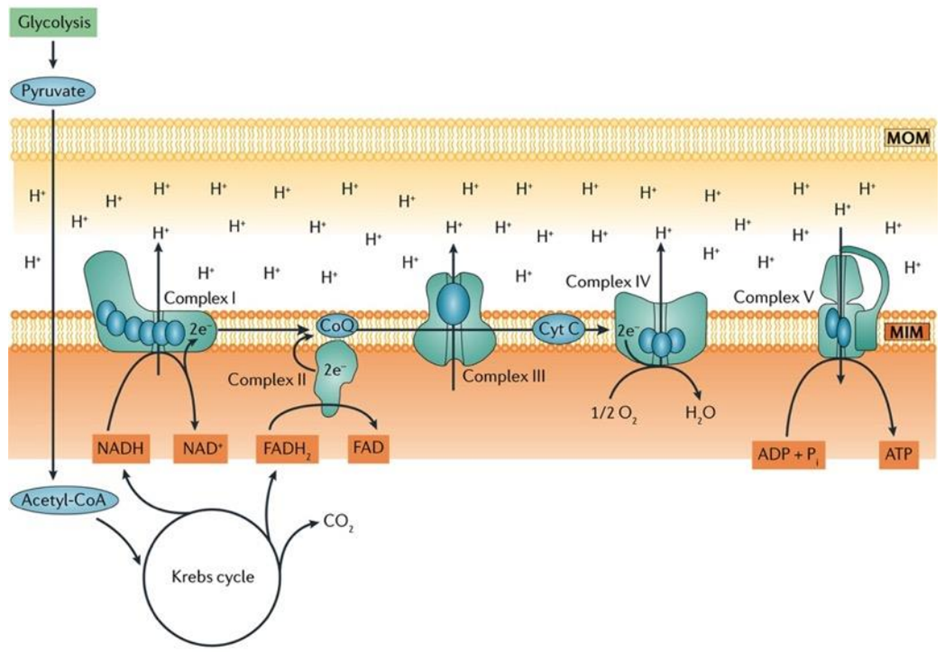
Fatty acids are activated into Acyl-CoA in the cytoplasm and are then transported to the mitochondria, where they are transformed into acetyl-CoA through beta-oxidation. This acetyl-CoA enters the TCA cycle, combining with oxaloacetate. Within the TCA cycle, substrate-level phosphorylation occurs once more, generating a small amount of ATP. Besides ATP production, the TCA cycle also produces NADH+H+ and FADH2, which act as carriers of electrons. As these electrons move from one complex to another, protons enter the intramembranous space. These protons subsequently pass through ATP synthase, causing it to rotate at a high speed, which facilitates the combination of ADP and Pi to form ATP.
This newly formed ATP combines with creatine, a compound synthesized by the liver from three amino acids (glycine, arginine, and methionine). This combination results in the formation of creatine phosphate, which is synthesized in the cytoplasm of myocardial cells from creatine and ATP. Importantly, it can be rapidly converted back into ATP during periods of elevated energy demand, such as during myocardial contraction. The enzyme creatine kinase, found in the myocardium, catalyzes the transfer of a high-energy phosphate group from creatine phosphate to ADP, effectively regenerating ATP. This process offers a swift source of ATP to support myocardial contractile function during times of increased heart rate or heightened stress.
Several animal species are known to be susceptible to cardiac diseases.
Dogs: Certain dog breeds, including Boxers, Doberman Pinschers, Great Danes, and Cavalier King Charles Spaniels, are predisposed to specific cardiac conditions like dilated cardiomyopathy (DCM) and mitral valve disease. DCM is characterized by the weakening and enlargement of the heart, making it less efficient at pumping blood, resulting in symptoms like fatigue, breathing difficulties, and fluid retention. Mitral valve disease involves a faulty closure of the valve between the left atrium and left ventricle, leading to blood leakage, which can cause heart chamber enlargement and symptoms such as coughing, breathing problems, and heart murmurs.
Cats: Hypertrophic cardiomyopathy (HCM) is a prevalent feline cardiac ailment, particularly affecting breeds like Maine Coon, Ragdoll, and Sphynx. HCM involves thickening of the heart’s walls, reducing its pumping efficiency, and resulting in symptoms like lethargy, breathing difficulties, and irregular heartbeats.
Horses: Horses can also suffer from cardiac diseases, including atrial fibrillation, valvular heart disease, and myocarditis. Atrial fibrillation refers to an abnormal heart rhythm affecting the atria.
Birds: Certain bird species, notably parrots and pigeons, can be vulnerable to cardiovascular diseases such as heart failure and atherosclerosis.
This article has been prepared from the presentation of our student, Doğa İsmailoğlu.
References
Heinrich Taegtmeyer (2012). Chapter 15 – Cardiomyocyte Metabolism: All Is in Flux, Editor(s): Joseph A. Hill, Eric N. Olson, Muscle, Academic Press, Pages 187-202, ISBN 9780123815101 https://doi.org/10.1016/B978-0-12-381510-1.00015-6.
Kodde IF, van der Stok J, Smolenski RT, de Jong JW (2007). Metabolic and genetic regulation of cardiac energy substrate preference. Comp Biochem Physiol A Mol Integr Physiol., 146(1):26-39. https://doi.org/10.1016/j.cbpa.2006.09.014
Kalp hastalıklarına Yatkın Irklar (2023). Veteriner Kardiyoloji. Available at:
http://kardiyoloji.veterinary.ankara.edu.tr/hangi-irk-hangi-kalp-hastaligina-yatkindir/
(Accessed: 27 October 2023).