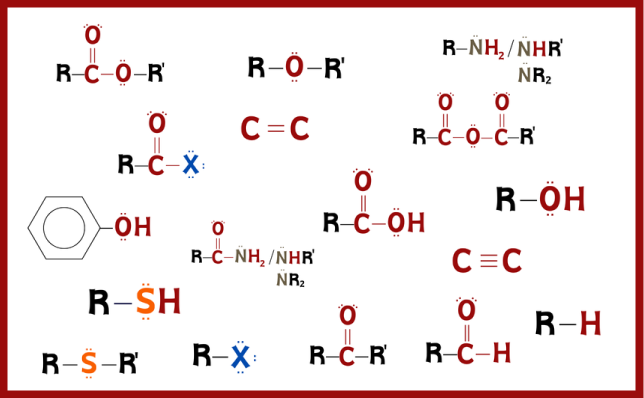
Organic chemistry is the key to understanding the molecular building blocks of life. Functional groups are the fundamental elements that organise this complex world. Ranging from carboxylic acids to alkanes, these groups determine the properties of organic compounds and determine the direction of chemical reactions. Carboxylic acids in particular play a critical role in vital processes such as esterification and acid-base reactions. Understanding functional groups is essential for understanding chemical reaction mechanisms and biochemical pathways.
Functional groups and the hierarchy between them provide guidance in understanding the basic rules of organic chemistry. This hierarchy is organised based on the chemical reactivity and priority of the groups. For example, carboxylic acids are ranked high because they have both high polarity and reactivity, while alkanes are generally simple hydrocarbons that are less chemically active. The order of these groups determines how compounds are named in the IUPAC nomenclature rules and is an important concept to learn for chemistry students.
This table categorises functional groups in detail for organic chemistry enthusiasts and provides information on their structures, nomenclature rules and example compounds. Organised under headings such as “Functional Group Class,” “Structure,” “Suffix-name,” “Prefix-name,” and “Example,” this table covers a wide range from carboxylic acid to alkane. For those who want to understand the properties and naming logic of functional groups, this table is both a visual and theoretical guide. You can examine this table to discover the cornerstones of organic chemistry and enrich your learning process!
Priority Order of Functional Groups
Subordinate Groups
Footnotes
- Sub-functional groups do not have a set priority. The functional group at the top of the list (carboxylic acid) has the highest priority for nomenclature, while the functional group at the bottom of the list (alkane) has the lowest priority for nomenclature.
- In organic chemistry, the symbol “R” is used as a general placeholder or abbreviation for any group to which a carbon or hydrogen atom is attached to the rest of the molecule.