All matter, such as solids, liquids, and gases, is composed of atoms. An atom is simply the smallest structural unit of matter, or the smallest part of an element that displays all its qualities is called an atom.
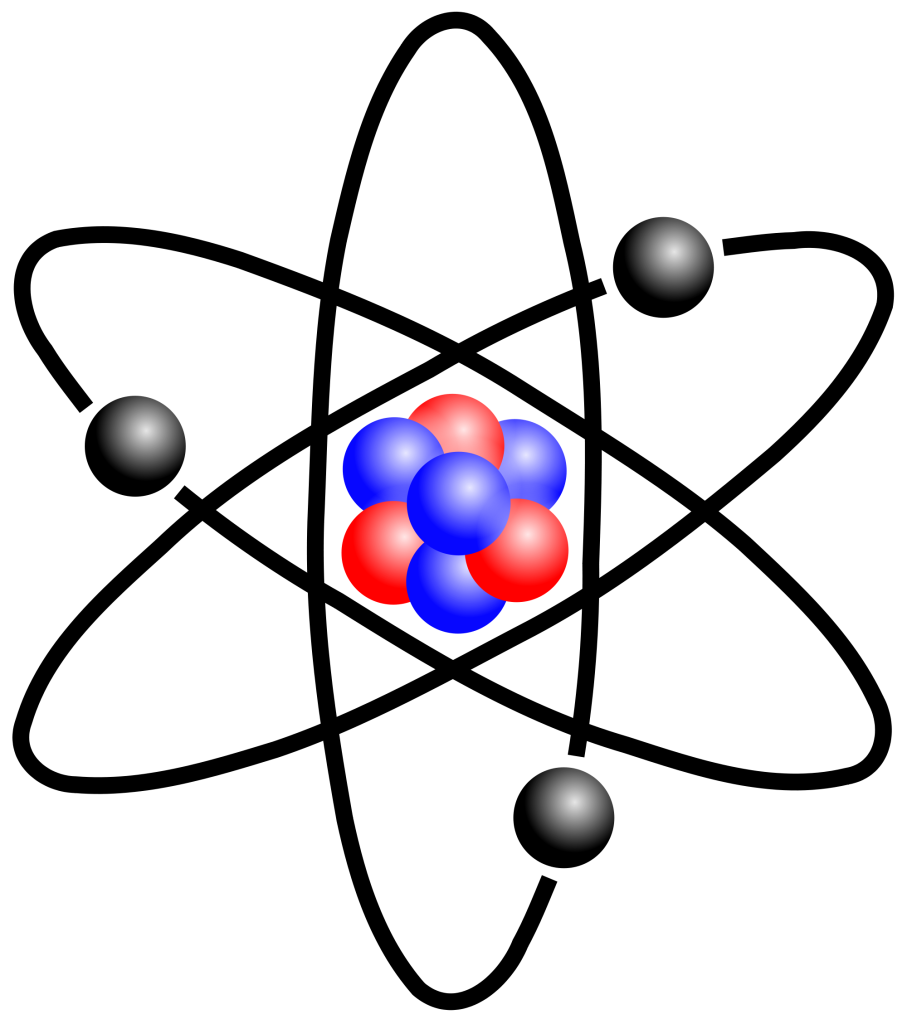
Atoms are the basic building blocks of matter. They are extraordinarily small, approximately spherical in shape. Their diameters can vary from 0.1 nm to 100 pm. Therefore, for the structure of matter, it is necessary to first define the atom and look at its properties. Atoms consist of a nucleus surrounded by electrons. The shell in which the electrons (electron shell) are located forms the basic volume of the atom. For an atom to be uncharged, the opposite charges of the nucleus and shell must be equal.
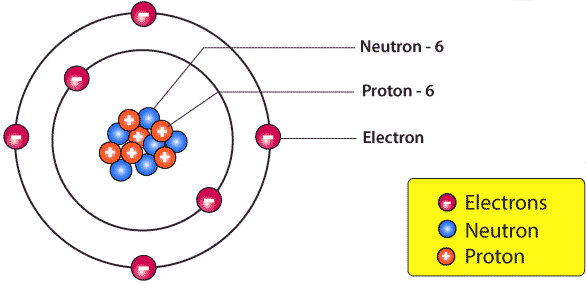
The nucleus consists of two separate particles. These are Protons and Neutrons. Almost equal masses of protons and neutrons builds up the nucleus.
Protons (p, H+, or 1H+) are positively charged while neutrons (n or n0) are uncharged. The mass of the electrons against the proton is 1/2000. This ratio is fixed and is not taken into account in the calculation of the atomic mass (atomic mass unit-amu) because the electron masses are very small. The mass of the nucleus is used directly in the calculation of the atomic mass unit.
Annotation
Atomic Weight or Atomic Mass?
Weight is defined as the force acting on an object by gravity with a unit of Newton (N), while the unit of mass is kilogram (kg) and is a quantity related to the amount or energy of matter. Mass is a measure of the inertia of matter.
According to Newton’s second law of motion, it describes the relationship between the mass of a substance and the amount of force required to accelerate it. This law is expressed by the equation F=ma. Here, “F” denotes the force acting on the substance, “m” the mass of the substance, and “a” denotes the acceleration of the substance. Weight is also a force, and if there is no other force acting on matter other than gravity, it can replace the force in the equation; Fw (force weight). In a non-accelerating item, the value a replaces gravity (g) (acceleration of gravity). Therefore, the equation F=ma changes to Fw=mg. Therefore, weight appears as another form of expression of the gravitational force.
The weight of a substance changes depending on the gravitational force of its location in the affecting space, but its mass does not change wherever the substance is.
In daily life, mass and weight are often used interchangeably. But we should know that they are different. Then we find the answer to the question in our title.
The simplest atom is the Hydrogen atom, which is chemically denoted by the symbol “H”. The nucleus of the hydrogen atom consists of a single proton, and the +1 charge of this proton is balanced by an electron in the electron shell. Phosphorus atom with the symbol P has 15 protons and 16 neutrons in its nucleus. The atomic shell has 15 electrons equal to the number of protons.
| Subatomic Particles | Symbol | Relative Electric Charge | Mass (g) | Relative Mass (amu) |
|---|---|---|---|---|
| Proton | p, H+, or 1H+ | 1+ | 1.6 * 10-24 | 1 |
| Neutron | n or n0 | 0 | 1.6750 * 10-24 | 1 |
| Electron | e− or β− | 1- | 9.1095 * 10-28 |
The number of protons in the nucleus of an atom, which has the same number of electrons as in the atomic shell, is defined as the nuclear charge number or atomic number (Z). It is also called Proton Number or Arrangement Number. Atomic number is unique to a particular element.
Nuclear Charge=Number of Protons=Number of Electrons
The sum of the number of protons and neutrons in the nucleus is also called Atomic Mass or Nucleon Number.
Isotopes
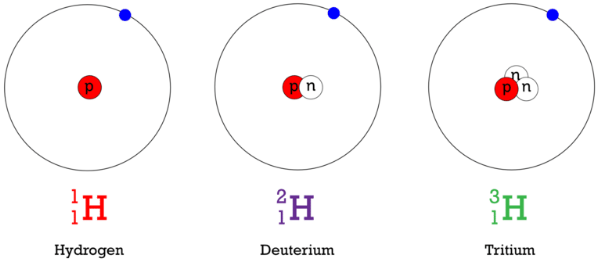
Atoms with the same number of protons form an element. Atoms with different atomic mass and the same chemical properties are called isotopes. In other words, isotopes have the same number of protons and different numbers of neutrons. Therefore, the difference between isotopes is due to the number of neutrons.
It is the number of electrons that determines the chemical properties of atoms. Isotopes of the same element have the same chemical properties (except for H) because they carry equal numbers of protons and therefore the same number of electrons. The physical properties of isotopes are different because the important determinant of physical properties is mass, and isotopes have different masses.
Some isotopes
- Isotopes of oxygen: Oxygen-16, Oxygen-17, Oxygen-18
- Uranium: U-235, U-238
- Chlorine: Cl-35, Cl–37
- Hydrogen: H–1 (Protium), H–2 (Deuterium), H–3 (Tritium)
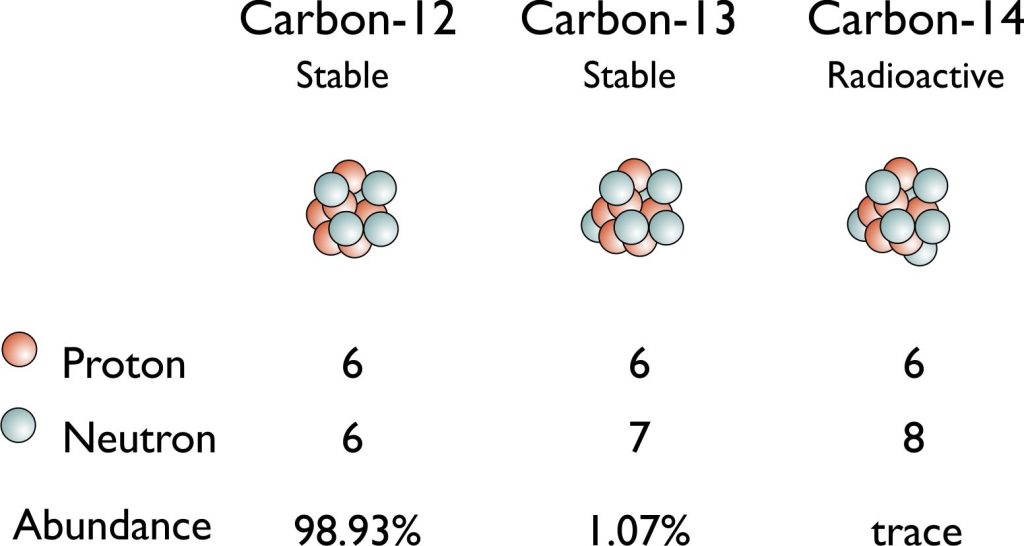
- Carbon: C–12, C–13, C–14 (Radiocarbon)
There are about 275 different isotopes of the 81 stable elements. There are more than 800 natural and synthetic radioactive isotopes available. A single element in the periodic table can have more than one isotopic form.
Carbon-12 (12C) and Carbon-13 (13C) are stable. Carbon-14 (14C) has the longest-half life. 12C is the most common carbon isotope in the world; about 98.93%. Atomic mass measurements are made in proportion to itself. The 13C isotope is found at a rate of 1.1% and important information about living things and the earth is obtained by making measurements in geology, paleoclimatology, paleoceanography sciences. The radiocarbon isotope (14C) is found especially in organic materials and the principal of radiocarbon dating is based on 14C (Williard Libby – Nobel Chemistry 1960). It is important in archeology and paleontology.
Isotopes can be found naturally, or they can be formed by giving additional neutrons to the nucleus by radiochemical methods. The added neutrons are not durable as they disrupt the proton:neutron ratio in the nucleus and cause fragmentation in the nucleus. This phenomenon is called radioactivity, and such isotopes are called radioisotopes. Radioisotopes give off their energy and become stable isotopes.
The breakdown of the nucleus of a radioactive atom by spontaneously emitting charged particles or rays is called radioactive decay. In other words, radioactive decay is the emission of energy in the form of ionizing radiation. This decay is related to the neutron:proton ratio in the nucleus. Each element has a specific neutron:proton ratio. Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissues and genetic materials.
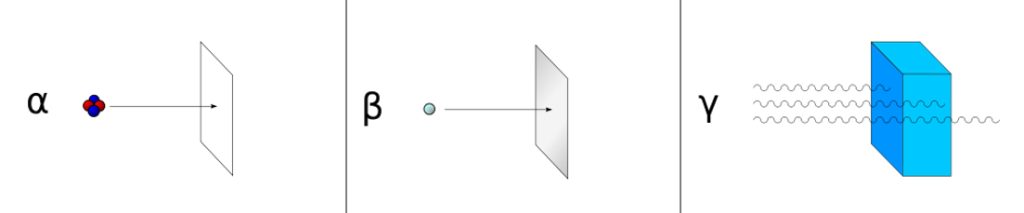
There are three types of energy lost through radioactive decay. These are α-Particles, β-Particles and γ-rays.
α-Particles or Alpha Rays or Alpha Radiation

and the mass number is reduced by 4.
The sequence in which radionuclides are formed one after the other, starting from a parent nuclide, is called the radioactive family or decay chain. The decay chain ends with a stable isotope of Pb or Bi. There are three types of natural degradation sequences, and components of the degradation sequence have different types of degradation and half-lives. These are the Uranium-Radium chain, the Uran-actinium chain, and the Thorium series.
β-Particles or β-ray or β-radiation
It consists of high-speed and energetic electrons or positrons emitted from some radioactive atomic nuclei. There are two types of beta-decay.
Negatron emission or electron emission (β-): It can be thought that it is formed as a result of the transformation of a neutron in the nucleus into a proton. With a β-radiation, the atomic number increases by 1, while the mass number does not.

Positron emission (β+): Positron emission is the emission from the nucleus of a particle with the same mass as the electron, but with a positive charge, which is formed by the conversion of a proton in the nucleus to a neutron, called a positron and represented by the symbol 0+1e (β+, o1 β).

Annotation
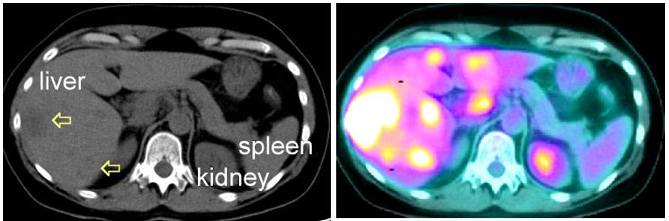
Positron emission tomography (PET)
PET is a modern nuclear medicine imaging technique. This technique allows some form of molecular imaging of a biological function in the body. PET images have a higher sensitivity than other imaging techniques. During PET imaging, (+) charged beta rays or positron (β+) emitting radiopharmaceuticals are administrated to the body. For example, 18F (Fluorodeoxyglucose) and 68Ga (Gallium-68) are common PET radiopharmaceuticals that emit β+ particles when degraded. With a PET scan, an image is obtained that highlights the cancer because the cancer has a higher metabolic rate than the surrounding tissues.
Reference: Demir M. Pozitron Emisyon tomografi (OET) Fiziği. Toraks Cerrahisi Bülteni 2015; 6: 146-53
γ-Rays
Gamma (γ) rays or gamma radiation are electromagnetic radiations with very short wavelengths, similar to X-rays, but rich in energy, which are emitted as a result of energy changes in the nucleus. A gamma ray or gamma radiation is a penetrating electromagnetic radiation resulting from the radioactive decay of atomic nuclei. It consists of photons in the highest observed photon energy range. Cosmic rays, nuclear fusion and experiments are sources of gamma rays.
Gamma rays and X-rays are both electromagnetic radiation, and they overlap considerably in the electromagnetic spectrum.
Gamma radiation also has widespread industrial use. For example, non-contact industrial sensors use gamma rays to detect measurement parameters such as the level, density and thickness of substances. Co-60 or Cs-137 isotopes are often used as radiation sources. These types of sensors are used in the mining, food and paper industries. In addition, gamma rays are used as an alternative to autoclave or chemical sterilization in the sterilization of medical equipment.
- Importance/use of radioisotopes in terms of biochemistry
- Research studies
- Determination of metabolites
- Diagnosis and treatment of certain diseases
- In field of drug developement and or to follow drug metabolism
- Age detection
- Sterilization of foodstuffs and laboratory materials
Radiation
The emission of radioactive materials such as alpha, beta, gamma and X- rays is called radiation, and the rays that are exposed affect living systems depending on their intensity and duration. Most affected cells and organs include lymphocytes, erythrocytes, gastrointestinal tract, eyes, anterior pituitary lobe, egg follicles, and mucous membranes.
Cosmic rays, gamma rays, beta particles, any form of alpha particles cause the formation of very reactive ions and even more reactive free radicals as they pass through the cells. These products can disrupt cellular activity, and in some cases, they destroy cells.
Radioactivity: It expresses the number of disintegration of a radioactive source per unit time. It refers to the state of an unstable atomic nucleus becoming stable by emitting electromagnetic or particle radiation.
Curie (Ci): One curie (1 Ci) is equal to 3.7 × 1010 radioactive decays per second, which is roughly the amount of decays that occur in 1 gram of radium per second and is 3.7 × 1010 becquerels (Bq). In 1975 the becquerel replaced the curie as the official radiation unit in the International System of Units (SI).
1 Ci= 3.7x 1010 Bq | 1Bq= 2.7x 10-11 Ci
Annotation
(7 November 1867 – 4 July 1934)
She was a Polish physicist and chemist (later citizen of France) who conducted pioneering research on radioactivity. She was the first woman to win a Nobel Prize, the first person to win twice, and the only woman to win a Nobel Prize in two different sciences, and was part of the Curie family’s five Nobel Prize legacy (1903 Physics, 1911 Chemistry).

Electron Shell
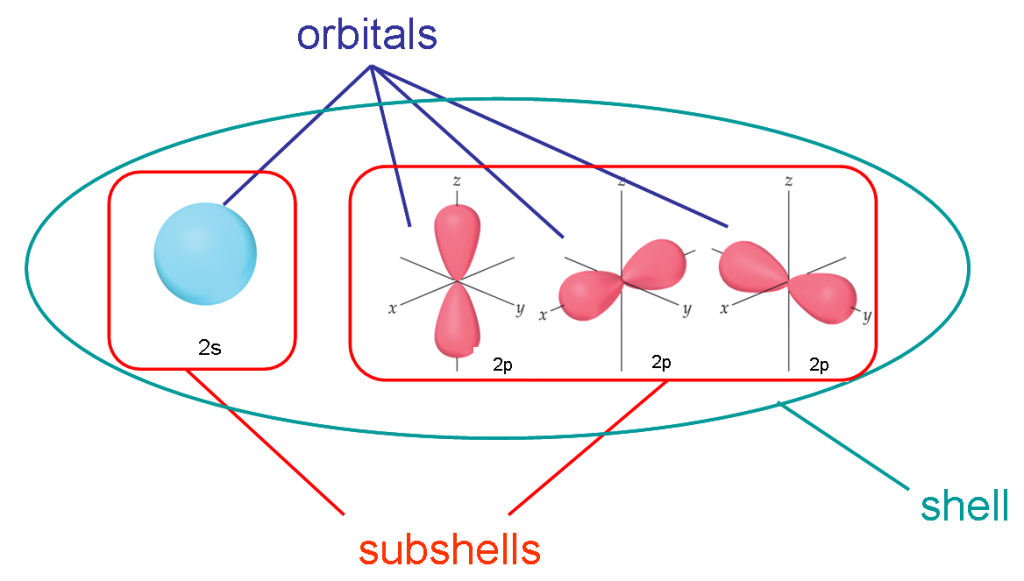
The orbitals in which an electron of a certain energy level is found around the atomic nucleus with a probability of 90% or more are defined as orbitals. It is possible to represent electrons as a cloud around the nucleus. Where clouds are dense, electrons are more likely to be found, and clouds are called orbitals. In addition, it is useful to explain the sometimes confusing concepts of shell, subshell, and orbital.
Shells: It is the path that electrons follow around the nucleus of an atom. These are also called energy levels because these shells are arranged around the nucleus according to the energy generated by an electron in that shell. The shell with the lowest energy is closest to the nucleus. The next energy shell is found beyond this layer. In other words, the energy levels of electrons increase from the inside out. The amount of energy is directly proportional to the shell number. Shells are named using letters K, L, M, N etc. The shell with the lowest energy level is the K layer, it contains at most 2*12=2 electrons.
Subshells: Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals. These are named according to the angular momentum quantum number. There are 4 main types of subshells that can be found in a shell. These are called s, p, d, f. Each subshell consists of several orbitals.
| Subshell | Number of orbitals | Maximum number of electrons |
|---|---|---|
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
Orbital: Orbital is a mathematical function that describes the location and wave-like behavior of an electron. The term orbital describes the complete motion of an electron. A subshell consists of orbitals. The number of orbitals in a subshell is a unique property. An orbital can only hold a maximum of two electrons. These electrons are in the same energy level, but differ from each other according to their spin. They always have opposite turns. When electrons are filled into orbitals, they are filled according to Hund’s Rule. This rule indicates that each orbital in a subshell is singly filled with electrons before any orbital pairs bond.
Since the electrons are all negatively charged, they are expected to repel each other, but on the contrary, they attract. This is explained by the spin properties of electrons. Accordingly, electrons revolve both around themselves and around the nucleus. However, since it is in the opposite direction of the spin axis of the neighboring electron around itself, an electromagnetic field is formed. This force of attraction is more than the force of repulsion.
Aufbau rule or principle states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. Therefore, orbitals are filled according to their increasing energy levels in. Electrons occupy the lower energy position first, then jump to higher energy levels only when the lower levels are filled. An orbital can have a maximum of two electrons. The spins (directions) of these electrons must be different (Pauli Exclusion Principle). Hund’s rule takes into account the placement of electrons in collapsing orbitals of the same subshells (s, p, d). Bonding to s, p, d subshells cannot occur unless and until each orbital is joined by an electron. Electrons repel each other because they are negatively charged. Repulsion can be reduced by separating them and placing them on indifferent degenerate subshells. All subshells with a single electron will spin in the same direction, either clockwise or counterclockwise. While the electrons fill the orbitals with the same energy, they first settle one by one in the same direction (spin), and then the number of electrons in the orbitals is doubled with the opposite direction electrons.
Bohr’s Model of the Atom
In 1913, Niels Bohr presented a model of the atom that characterized an atom as a small, positively charged nucleus surrounded by electrons orbiting the positively charged nucleus in circular orbits, comparable to planets orbiting the sun in our solar system with the attraction provided by electrostatic forces. The Bohr model of an atom is a popular name for this model. The atomic model of a hydrogen atom was proposed by Bohr. The stability of orbiting electrons was neatly explained by Bohr’s model. These orbitals were called “energy shells” by him. It is the classical atomic model that describes electrons as negative charges surrounding the nucleus in the form of dots.
Annotation
(7 October 1885 – 18 November 1962)
Niels Bohr was a Danish physicist who made fundamental contributions to the understanding of atomic structure and quantum theory, for which he was awarded the Nobel Prize in Physics in 1922. Bohr was also a philosopher and supporter of scientific research.

Edited on: 29 September 2023




